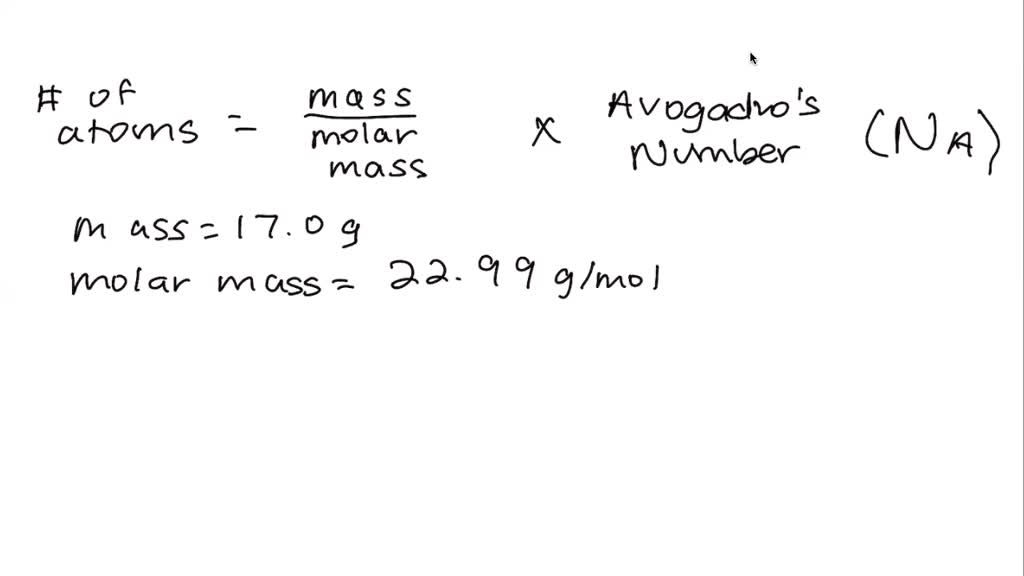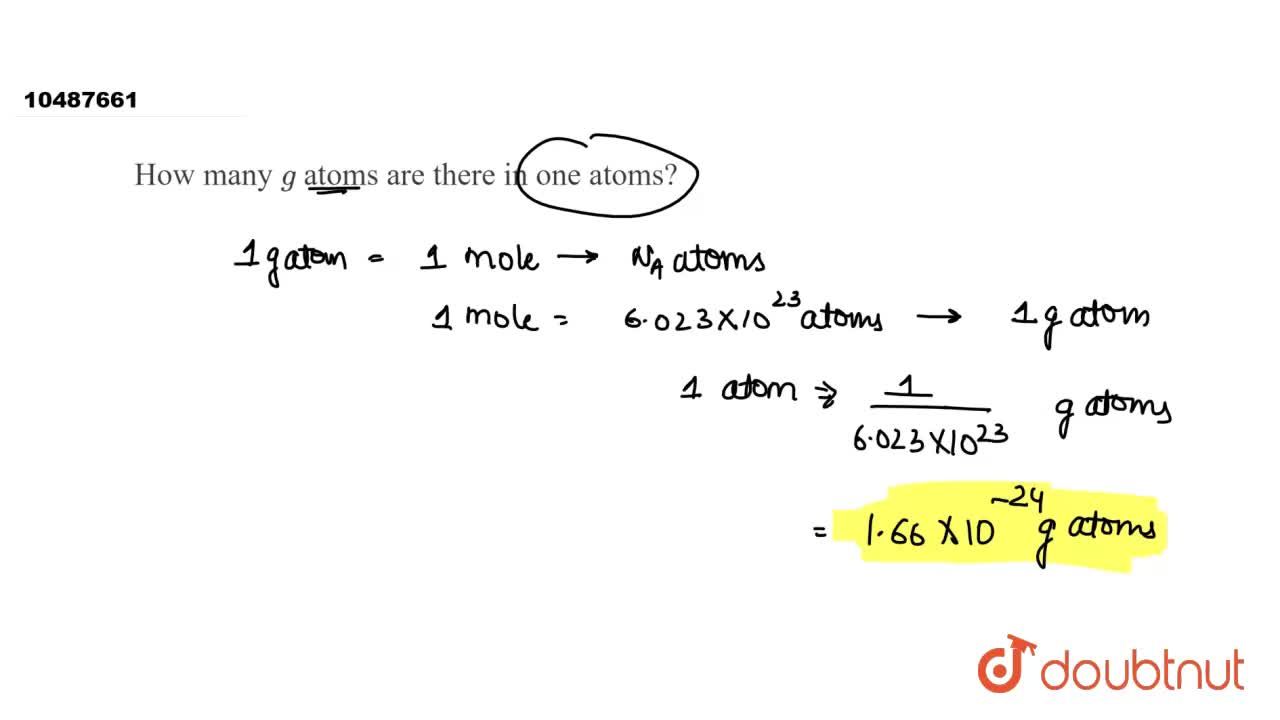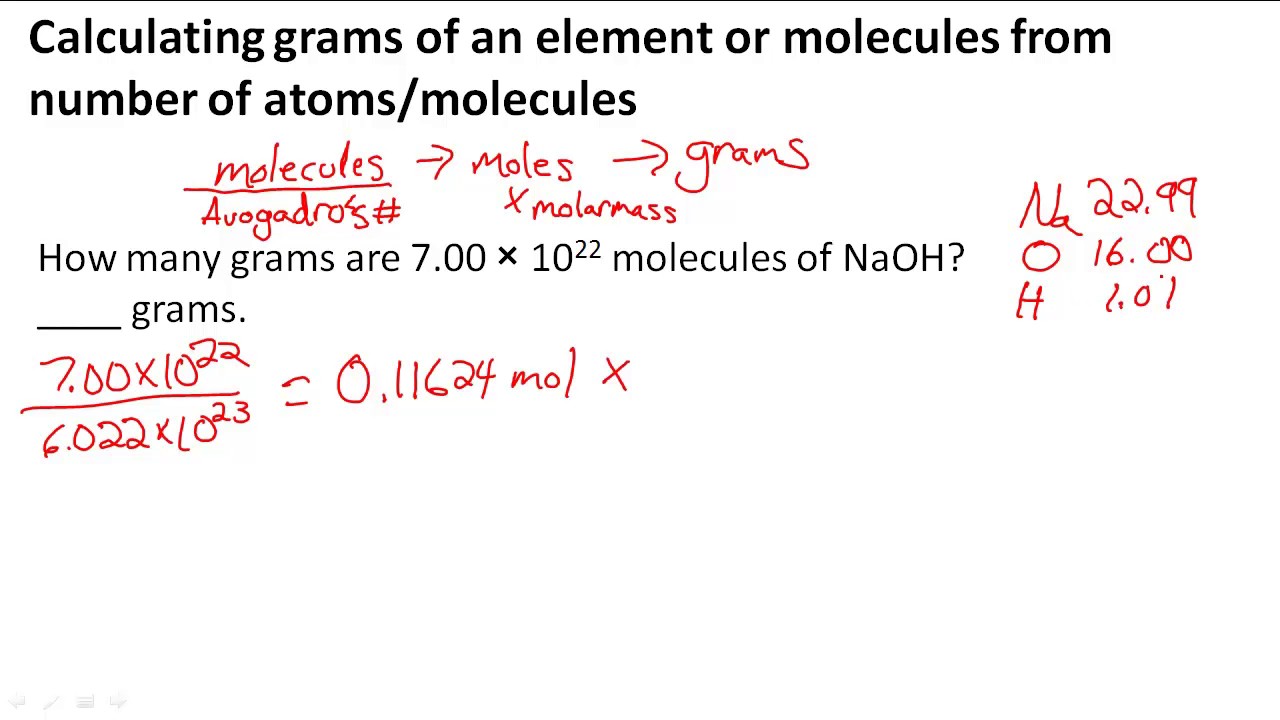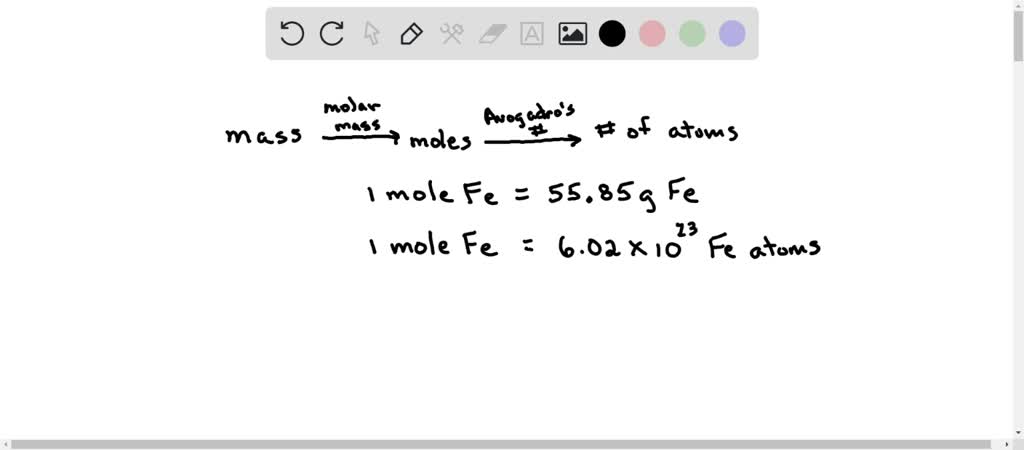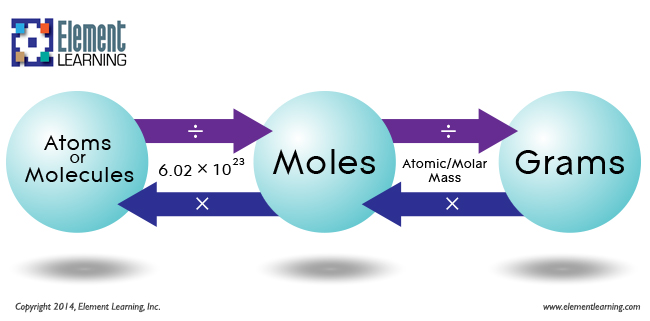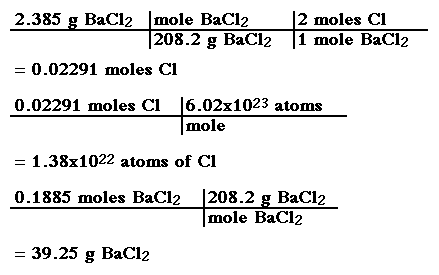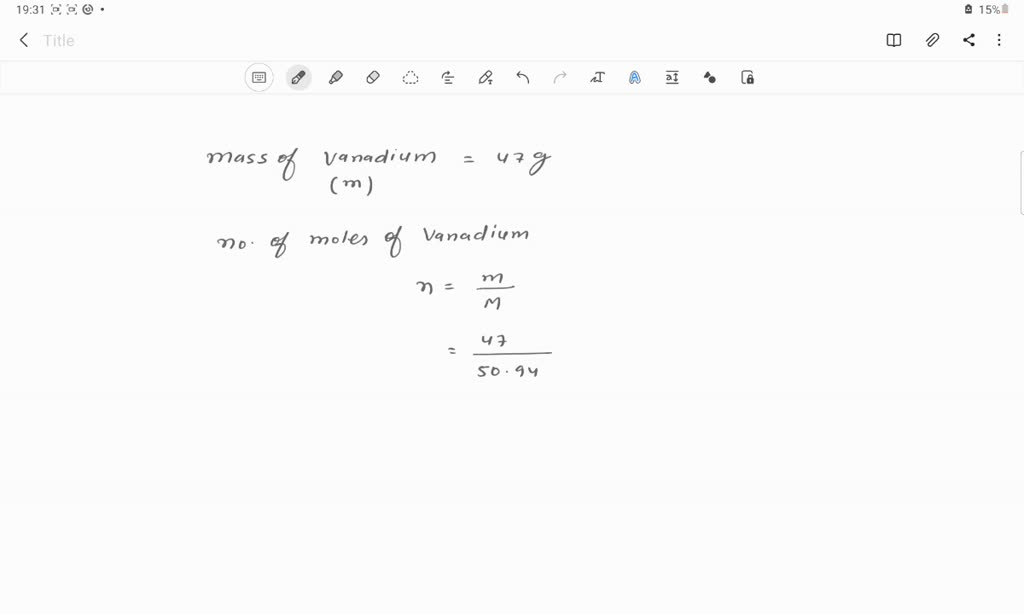
SOLVED: Determine the number of atoms in 47.0 grams of vanadium, V. (The mass of one mole of vanadium is 50.94 g.)

1. Using Avogadro's number, calculate the number of atoms in 0.005 kilograms of carbon. 2. If there are 'x' atoms in 5 grams of carbon, how many atoms are there in 5

Calculate the number of atoms in each of the following:(i) 52 moles of Ar (ii) 52 u of He (iii) 52 g of He

1. Using Avogadro's number, calculate the number of atoms in 0.005 kilograms of carbon. 2. If there are 'x' atoms in 5 grams of carbon, how many atoms are there in 5